
TYBER WAY
Bringing Products
To Market Faster
We leverage our focus on rapid commercialization and bioengineered technology platforms to cut through the red tape so you can start selling and focus on your next big idea.

- Cervical Interbody
- Lumbar Interbody
- Cotton Wedge
- Evans Wedge
- PEEK Hammetoe

- HD HL Screws
- PEEK Screws
- Snap-Off Screw
- MIS Bunion Screws
- Metal Hammertoe
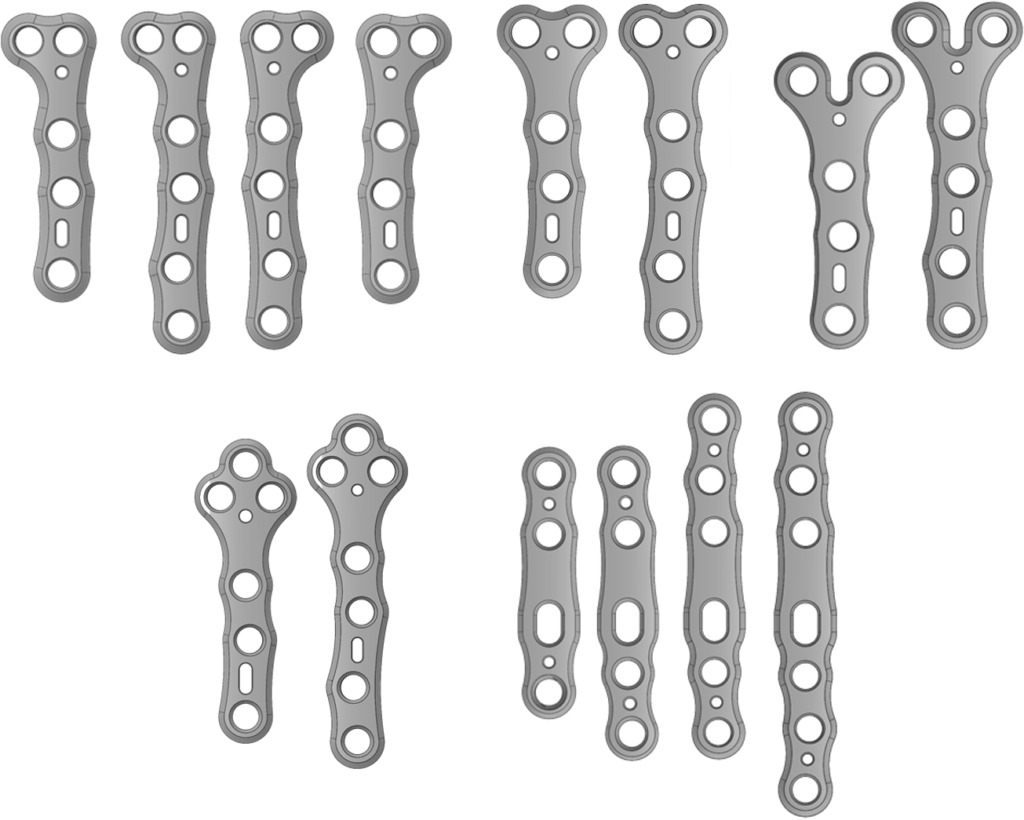
- Lower Extremity
- Upper Extremity
- Proximal Tibia
- Mini Fragment
- Small Fragment

- Nitinol Staple
- Sterile Kits
- Implantable k-wires
- Sterile / Non-Sterile
- Fibular Nail
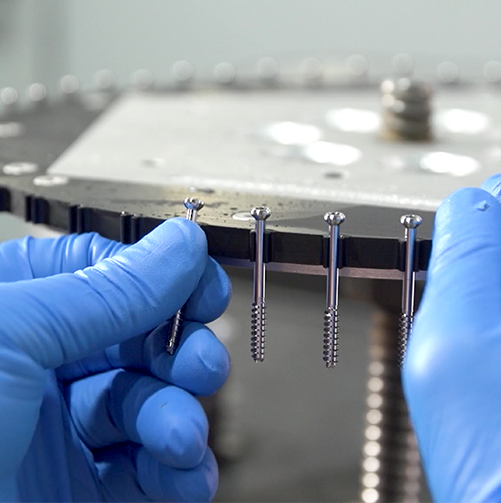
Fill Portfolio Gaps
Our regulatory-cleared products are ready for your private label.

Reduce Time to Market
Experience the value of Tyber Medical and start selling your product sooner.

Expand Your Resources
Leverage our industry expertise to amplify your team’s capabilities.

Update Your Devices
Revitalize your products with industry-leading, innovative orthopedic solutions.
Capabilities
Research and Development
We are dedicated to creating advanced medical devices through research and advanced iterative design techniques.
Quality Management
Our robust processes and checks and meticulous handling of required documentation ensures product excellence.
Supply Chain Management
This strategic approach not only allows for precise oversight but also directly boosts our on-time delivery rate to over 95%.
Regulatory Clearances
This facilitates smoother market access and bridges the gap between our innovative solutions and patients worldwide.

Manufacturing
The vertically integrated operations at our two state-of-the-art manufacturing facilities drives our unparalleled levels of quality while ensuring consistent on-time delivery.
Microbiology
Our lab is dedicated to pioneering efforts in research and development, conducting additional tests that propel advancements in the field.

Clinical Research
Through rigorous clinical trials and studies, we gather data on the safety, effectiveness, and potential applications, ensuring they meet the real needs of patients, practitioners, EU MDR, and FDA.
Commercial Support
From the conception of an idea to its successful launch and beyond, Tyber’s Commercial Support ensures that innovations are not only seen and accessible but also make a lasting impact in the medical world.
